There is an ongoing debate about certain national and international policy initiatives concerning the mandatory licensing of COVID-19 vaccine patents and we see the need to clarify certain concepts. In the event of mandatory licensing, it is more than likely that the necessary manufacturing capacity, production conditions and health guarantees would not be met. Indeed, […]

As we all know, on 1 January 2021 the new version of the Code of Practice for the Pharmaceutical Industry (the ‘new Code’ or, simply, the ‘Code’) came into force. As well as incorporating the amendments introduced in June 2019 to the Code of Practice of the European Federation of Pharmaceutical Industries and Associations (EFPIA), […]

In light of the current COVID-19 health crisis, it is an apt moment to highlight the importance of intellectual property. Intellectual and industrial property, among others, allow us to turn ideas into concrete projects that create value, wealth, jobs, and cover unmet medical needs. Above all, intellectual property serves as a basis for societal progress […]

Interruption of supply or the withdrawal of medicines from the market and competition rules. Henar González Durántez y Pilar Carrasco del Olmo 2021 versión of Pharmaceutical Industry Code of Best Practices. Teresa Paz-Ares Rodríguez, Beatriz Cocina Arrieta y Nuria Porxas Roig Regulatory framework aplicable to observational studies with medicines for human use foreseen […]

Recent investigations of the pharmaceutical sector by the competition authorities demonstrate a focus on examining a number of actions that could entail an interruption of supply or even the withdrawal of medicinal products from the market by pharmaceutical companies. Specifically, in the field of pharmaceutical law, the competition authorities have devoted particular attention to two […]

On 19 January CEFI organised a seminar to deepen our understanding of vaccines in times of pandemic. Interest in the debate, reflected in the number of registrations (298) and the quality of the speakers, most of whom are at the forefront of work to provide a vaccine for the current health crisis, ensured the session […]

Fascinating debate on vaccines with 266 registered participants and fantastic presentations by the speakers.

On 19 January 2021, CEFI will hold the online conference entitled Deepening our understanding of vaccines in times of pandemic. Registration is free of charge. Programme and registration

Two new issues of the journal published by CEFI are available: Cuadernos de Derecho Farmacéutico issue 75 (October-December 2020). Comunicaciones en Propiedad Industrial y Derecho de la Competencia issue 91 (September-December 2020).
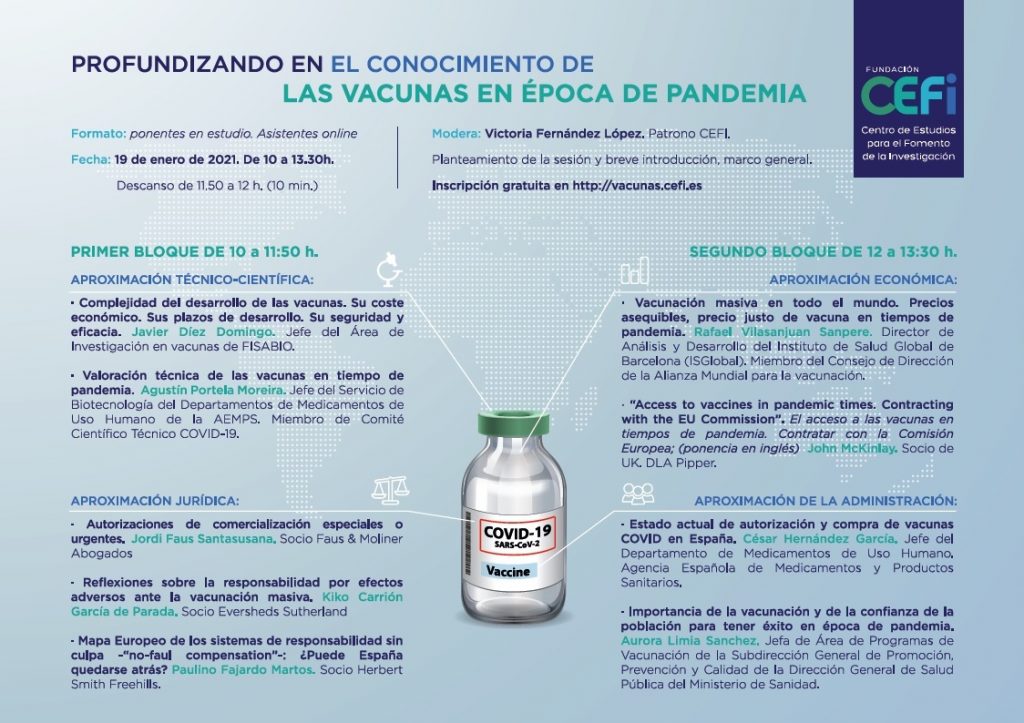
On 19 January 2021, CEFI will hold an online conference entitled Deepening our understanding of vaccines in times of pandemic. Programme and registration

There have been some eventful days recently in terms of important pharmaceutical regulations and legislation: Community pharma strategy to which we dedicated a post on LinkedIn Proposal for a Data Governance Regulation. Re-use of certain public sector data, including health data, which is key to boosting biomedical research. RD on observational studies. Simplifying administrative procedures, […]

Global action in the fight against the COVID-19 pandemic. Joint statement by the International Coalition of Medicines Regulatory Authorities (ICMRA) and the World Health Organization (WHO), supported by the Spanish Medicines and Health Products Agency (AEMPS), with regulatory agencies adopting a series of measures to promote alignment on regulatory processes across all countries. Global collaboration, […]












